Abstract
Background: CD19 CAR T cells have demonstrated high response rates in patients (pts) with relapsed or refractory (R/R) lymphoma, but these therapies are associated with high rates of cytokine release syndrome (CRS) and immune effector cell-associated neurotoxicity syndrome (ICANS), often requiring prolonged hospital stay including transfer to the intensive care unit (ICU). Real-world data and early and prophylactic corticosteroid trials with axicabtagene in R/R large cell lymphoma have reported lower rates of severe CRS to <10%, but severe ICANS remains elevated at 13-31% (Nastoupil L et al. J Clin Oncol2020; Oluwole O et al. BJH 2021). We have previously reported that elevated interleukin-1 (IL-1) in cerebrospinal fluid (CSF) was associated with ICANS (Santomasso B et al. Cancer Discov 2018), and IL-1 inhibition prevented development of severe ICANS and CRS in the in vivo preclinical model (Giavridis T et al. Nat Med 2018). Based on these data, we initiated a phase II study of IL-1 receptor inhibitor, anakinra (SOBI), in adult pts receiving commercial CD19 CAR T cells for prevention of CRS and ICANS (NCT04148430).
Methods: Adult pts with R/R large B-cell lymphoma (LBCL) and mantle cell lymphoma (MCL) receiving commercially available CD19 CAR T cells were enrolled. Pts received anakinra 100mg subcutaneous every 12 hours starting either on day 2 of CAR T cell infusion or after 2 documented fevers of ≥38.5 prior to day 2, whichever was earlier. Anakinra was continued for a minimum of 10 days, and the dose could be increased to 100mg every 6 hours and continued beyond 10 days in the case of persistent or progressing CRS and ICANS. Pts received tocilizumab and/or corticosteroids after anakinra initiation for persistent or worsening CRS or ICANS. CRS and ICANS were assessed per the ASTCT consensus grading. Disease response was assessed per the Lugano criteria. The primary objective was to determine the rate of severe ICANS within the first 28 days of CAR T cell infusion. Secondary objectives included assessment of severe CRS, all grades of CRS and ICANS, disease response and serum and CSF cytokines.
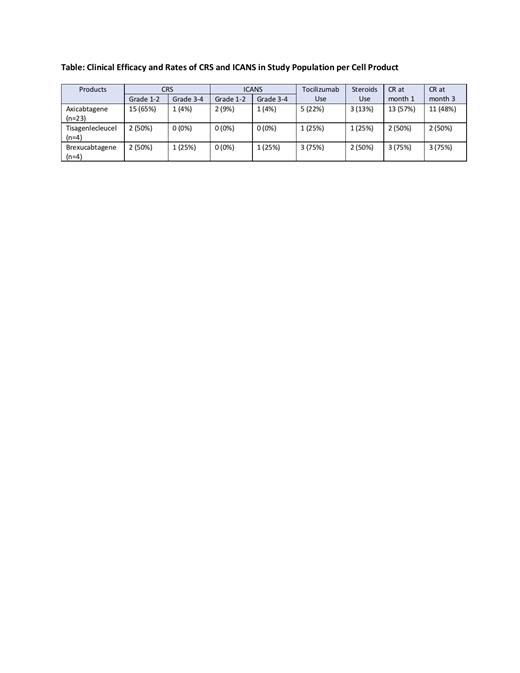
Results: A total of 31 pts (LBCL=27, MCL=4) were enrolled to the study. The median age of the pts at the time of T cell infusion was 62 (range, 30-77). CD19 CAR products included axicabtagene (23 pts; 74%), tisagenlecleucel (4 pts; 13%) and brexucabtagene (4 pts; 13%). 17 pts (55%) received bridging therapy prior to T cell infusion. All pts started anakinra at 100mg q12h; 25 pts started on day 2 and 6 pts prior to day 2 for grade 1 CRS. Anakinra dose was increased to 100mg q6h in 13 pts (42%). The median duration of anakinra administration was 10 days (range, 10-27). CRS of all grades was observed in 21 pts (68%) with severe CRS (grade 3-4) in 2 pts (6%) (1 with axicabtagene and 1 with brexucabtagene) (Table). Median CRS duration was 5.5 days (range, 1-21). ICANS of all grades was observed in 4 pts (13%), with severe ICANS in 2 pts (6%) (both grade 3; 1 with axicabtagene and 1 with brexucabtagene). No pt experienced grade 5 CRS or ICANS. Tocilizumab and corticosteroids were used in 9 pts (29%) and 6 pts (19%), respectively. Three pts (10%) required ICU transfer (2 with brexucabtagene and 1 with axicabtagene). In LBCL pts treated with axicabtagene (n=23), both severe CRS and ICANS were observed in 4% with tocilizumab and steroid use in 22% and 13%, respectively (Table). With a median follow-up of 104 days (range, 21-363), overall disease response rate at month 1 was 74% for all pts, with CR rate at 1 and 3 months of 58% and 52%, respectively.
Conclusion: Early use of IL-1 receptor inhibitor anakinra appears to be safe and feasible, and reduces the rates of both severe CRS and ICANS with the comparable response rates in adult pts with R/R B-cell lymphoma receiving CD19 CAR T cells. The overall severe CRS and ICANS rates were 6% each with relatively low utilization of tocilizumab (29%) and corticosteroids (19%). In pts receiving axicabtagene, the rate of severe ICANS was 4%. A longer follow-up is needed to assess durability of remission, but the study provides strong support for continued investigation of IL-1 inhibition in prevention of severe ICANS. Further exploratory analysis is planned to address the impact of anakinra on changes in serum and CSF cytokines.
Park: BMS: Consultancy; Servier: Consultancy; Minerva: Consultancy; Curocel: Consultancy; Autolus: Consultancy; PrecisionBio: Consultancy; Intellia: Consultancy; Kite Pharma: Consultancy; Amgen: Consultancy; Artiva: Consultancy; Kura Oncology: Consultancy; Novartis: Consultancy; Affyimmune: Consultancy; Innate Pharma: Consultancy. Sauter: Gamida Cell: Consultancy; GSK: Consultancy; Bristol-Myers Squibb: Research Funding; Precision Biosciences: Consultancy; Kite/Gilead: Consultancy; Celgene: Consultancy, Research Funding; Genmab: Consultancy; Novartis: Consultancy; Spectrum Pharmaceuticals: Consultancy; Juno Therapeutics: Consultancy, Research Funding; Sanofi-Genzyme: Consultancy, Research Funding. Palomba: Juno: Patents & Royalties; Seres: Honoraria, Other: Stock, Patents & Royalties, Research Funding; Notch: Honoraria, Other: Stock; Novartis: Consultancy; Kite: Consultancy; Wolters Kluwer: Patents & Royalties; PCYC: Consultancy; Ceramedix: Honoraria; Lygenesis: Honoraria; Magenta: Honoraria; BeiGene: Consultancy; WindMIL: Honoraria; Rheos: Honoraria; Nektar: Honoraria; Priothera: Honoraria; Pluto: Honoraria. Shah: Amgen: Research Funding; Janssen Pharmaceutica: Research Funding. Dahi: Gilead sciences: Membership on an entity's Board of Directors or advisory committees; Kite pharma: Membership on an entity's Board of Directors or advisory committees. Scordo: Angiocrine Bioscience: Consultancy, Research Funding; Omeros Corporation: Consultancy; Kite - A Gilead Company: Membership on an entity's Board of Directors or advisory committees; i3 Health: Other: Speaker; McKinsey & Company: Consultancy. Batlevi: Kite Pharma: Consultancy; TouchIME: Honoraria; Seattle Genetics: Consultancy; Juno/Celgene: Consultancy; Karyopharm: Consultancy; TG Therapeutics: Consultancy; ADC Therapeutics: Consultancy; Medscape: Honoraria; BMS: Current holder of individual stocks in a privately-held company; Moderna: Current holder of individual stocks in a privately-held company; Regeneron: Current holder of individual stocks in a privately-held company; Viatris: Current holder of individual stocks in a privately-held company; Pfizer: Current holder of individual stocks in a privately-held company; Dava Oncology: Honoraria; Life Sciences: Consultancy; Memorial Sloan Kettering Cancer Center: Current Employment; Bayer: Research Funding; GLG Pharma: Consultancy; Xynomic: Research Funding; Roche/Genentech: Research Funding; Novartis: Research Funding; Epizyme: Research Funding; Janssen: Research Funding; Autolus: Research Funding. Perales: Merck: Honoraria; Bristol-Myers Squibb: Honoraria; Celgene: Honoraria; Equilium: Honoraria; Servier: Honoraria; Miltenyi Biotec: Honoraria, Other; MorphoSys: Honoraria; Takeda: Honoraria; Medigene: Honoraria; Incyte: Honoraria, Other; Cidara: Honoraria; Karyopharm: Honoraria; Kite/Gilead: Honoraria, Other; Nektar Therapeutics: Honoraria, Other; Sellas Life Sciences: Honoraria; NexImmune: Honoraria; Omeros: Honoraria; Novartis: Honoraria, Other. Santomasso: Janssen: Consultancy; Legend: Consultancy; Kite/Gilead: Consultancy; Celgene/BMS: Consultancy; Incyte: Consultancy; In8bio: Consultancy. Sadelain: Juno Therapeutics: Patents & Royalties; NHLBI Gene Therapy Resource Program: Other: Provision of Services (uncompensated); Minerva Biotechnologies: Patents & Royalties; Mnemo Therapeutics: Patents & Royalties; Fate Therapeutics: Other: Provision of Services (uncompensated), Patents & Royalties; Takeda Pharmaceuticals: Other: Provision of Services, Patents & Royalties; St. Jude Children's Research Hospital: Other: Provision of Services; Ceramedix: Patents & Royalties; Atara Biotherapeutics: Patents & Royalties. Brentjens: BMS: Consultancy, Patents & Royalties, Research Funding; Gracell Biotechnologies, Inc: Consultancy, Ended employment in the past 24 months; sanofi: Patents & Royalties; Caribou: Patents & Royalties.
Anakinra for prevention of CRS and ICANS associated with CD19 CAR

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal